Table of Contents
Example P21: Precipitation simulation during continuous casting of micro-alloyed Fe-Al-C-Cu-Mn-N-Nb-S-Si-Ti steel
Compatibility
MatCalc version: 5.44.0026
Database: mc_fe.tdb, mc_x_FeAlCCuMnNNbSSiTi.tdb,
mc_sample_fe.ddb
Author: G. Stechauner
Created: 2011-12-22
Revisions:
Objectives
A more complex precipitation system compared to P20 was selected for this example. This example features the simultaneous precipitation of two different precipitates. It is important to mention, that every element needs a phase where it can nucleate. Therefore Cu2s was chosen for the copper, and MnS_Q for the sulfur. If an element has no phase where it can be, massive errors can occur (e.g.: liquid phase becomes stable at 300C, etc…).
This example starts with a Scheil calculation to determine the phase fractions of both precipitates. It then continues with the virtual treatment to set up the primary precipitates.
With this method, an adequate calc state was found, which can be used for further precipitation calculations. However these will not be performed in the course of this example, but you are still encouraged, to play around with MatCalc similar to P20-2.
Related documents
- P20 - Precipitation simulation during continuous casting of micro-alloyed Fe-Al-C-N-Nb-Ti steel - Part I is similar to this example, Part II can be used as a basis to play around.
Complementary files
Click here to view the script for the Scheil calculation to estimate the phase fractions.
Click here to view the script for the calculation of the primary precipitates.
Main document
Consider the same microalloyed steel as in example E20. The following table summarizes the chemical composition of the steel used in this study. Nominal composition given in wt%.
| C | Al | Cu | Mn | N | Nb | S | Si | Ti |
|---|---|---|---|---|---|---|---|---|
| 0.4 | 0.03 | 0.05 | 0.5 | 0.01 | 0.035 | 0.01 | 0.3 | 0.01 |
Setup thermodynamics
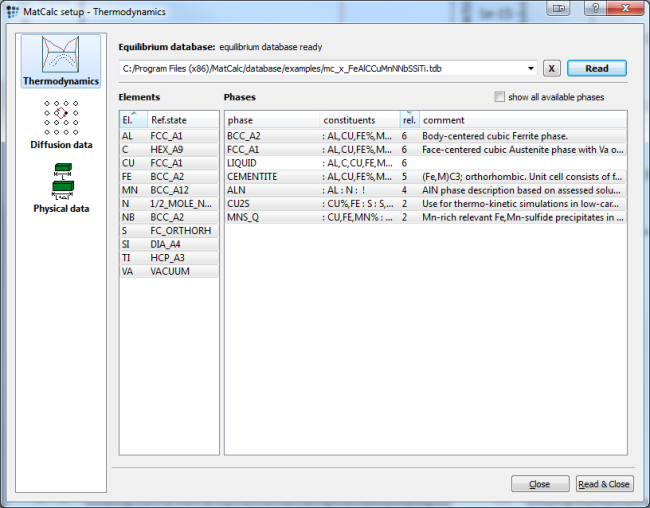
For precipitation simulation, either the 'mc_fe' or the 'mc_x_FeAlCCuMnNNbSSiTi' database can be used. Select all elements and phases but the liquid one when using the example database, select the highlighted ones when using mc_fe. Include liquid phase for Scheil calculation:
After reading the database as shown above, load the diffusion database for Fe, as it is needed in precipitation simulation. Then enter the composition of the system in the 'Composition …' dialog of the 'Global' menu according to the table shown above.
Setup phases
As we need an equilibrium phase of FCC_A1 later, we want to change the automatically created FCC_A1#01 complex Carbo-nitride phase to an equilibrium phase. To do so, we simply change the major constituents of the FCC_A1#01 phase in the phase status dialog window to :FE:VA: and suspend it.
To still account for the carbo- and nitride phase, we use composition set to create the NbC and TiN. Highlight the FCC_A1 and create a new composition set. Enter :NB%,TI:C%,N,VA: as the constituents for the NbC phase, and :TI%,NB:N%,C,VA: for the TiN phase respectively.
Set start values and calculate an equilibrium at 1500C.
Important note …
Evaluate phase fraction of primary precipitates
Perform a Scheil-Gulliver analysis to obtain the values for phase fraction and chemical composition of precipitation phase. Refer to E20 if you want to refresh your knowledge about this method.
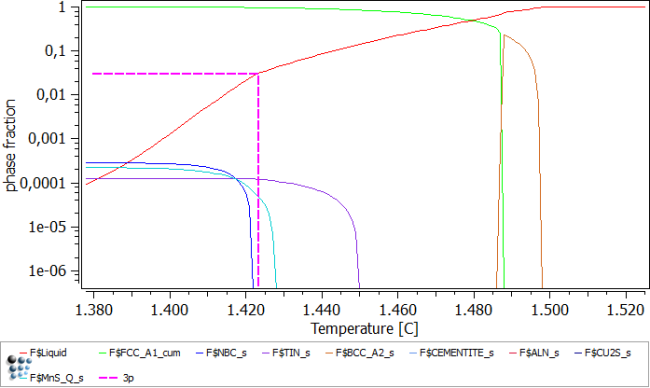
Start off by setting the automatic start parameters and calculating an equilibrium aut 1500C, to get a starting point. Choose a temperature of 2000C to be for sure in the liquid phase, allow back-diffusion of C, N and S and calculate to a minimum liquid fraction of 3% (enter as 0.03). When asked, allow to create the Scheil phases.
Important note …
Now we need to impose the δ-ferrite to γ-ferrite transformation. To do so, open the 'Transformation' window, and add a new transformation. Transform BCC_A2_S to FCC_A1_S and use FCC_A1_T as the equilibrium phase (Note: Enter 'From' and 'To' phase, then press 'Create equilib phase …' button to create FCC_A1'T). Back in the Scheil calculation dialog box, leave all settings as they were but impose to newly created transformation. Proceed with calculating and plotting the results.
The easiest method to evaluate the phase fractions is to open up the Buffer states window (Ctrl + L) and select the temperature, corresponding to 3% residual liquid. This is the temperature at 1423C.
Phase fractions obtained at 1423C:
#### /LIQUID/ moles: 0,0309315, gm: -101315 (-101315), sff: 1 Phasestatus: entered - active FE +9,10756e-001 C +4,94093e-002 SI +1,81992e-002 MN +1,34109e-002 NB +4,58825e-003 S +1,23130e-003 CU +9,29529e-004 N +5,48362e-004 TI +4,79867e-004 AL +4,46806e-004 #### /TIN_S/ moles: 0,000121085, gm: -224779 (-224779), sff: 0,500199 Phasestatus: fixed dfm: -4,03974e+001 TI +4,94454e-001 N +4,47215e-001 C +5,25861e-002 NB +5,74425e-003 #### /MNS_Q_S/ moles: 5,25007e-005, gm: -167279 (152199), sff: 0,5 Phasestatus: fixed dfm: -1,50948e-001 S +5,00000e-001 MN +4,83805e-001 FE +1,60690e-002 CU +1,26132e-004
Now that we know the phase fraction and chemical composition of the primary precipitates, we can start to build our virtual heat treatment.
Setting up the precipitation system
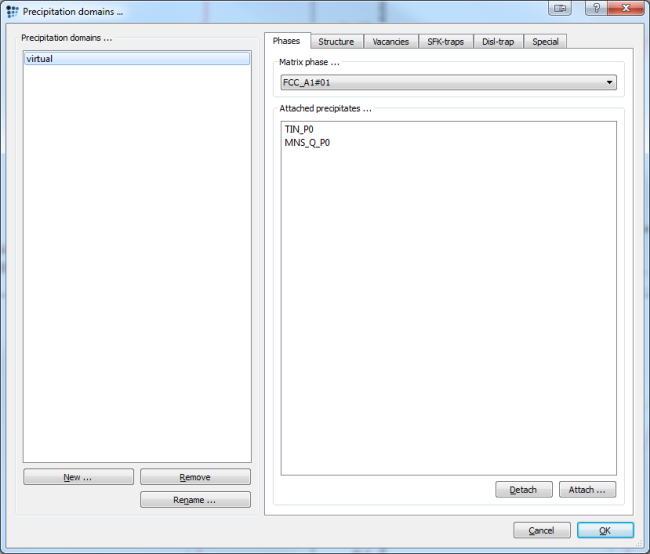
To proceed with the precipitation kinetics calculation, you could either start a new instance of MatCalc, or delete all the Scheil and transformation phases in the current one. Before starting with the following part, check your phases status and chemical composition to make sure, everything is correct. If not, set the system up anew as described in the system setup section without creating Scheil phases. Now open the precipitation domain dialog window and create a domain for the virtual treatment and select the beforehand created equilibrium phase FCC_A1#01 as its matrix phase.
Continue by defining the precipitates:
- Bring up the phase status dialog
- Create two precipitates, one for TiN and one for MnS_Q
- Initialize both precipitates with 25 size classes
- Set the precipitation sites to dislocations
- Restrict the nucleation to valid major constituents and to the precipitation domain 'Virtual'
Setting up the virtual heat treatment
As already mentioned in the 'HowTo' manual, the values which are used in this step, are absolutely arbitrary. If a heating rate of 10000K/s followed by annealing for 100 years at 1000C leads to the desired size and number density of the primary precipitates, that's fine! The only limitation one should keep in mind, is the correctness of the obtained values, and the minimization of processing time. Minimization of processing time can be achieved by ensuring that no radius shrinking takes place. If this happens, initialize the system with a smaller number density. Because primary precipitates are rather big, we are trying to achieve a size of a few µm, with a phase fraction and chemical composition similar to the Scheil analysis.
To get an idea, at which temperature to anneal to achieve the desired phase fraction, make an equilibrium calculation before creating the precipitates, or suspend them if you already created them. A temperature of about 1402C showed a MnS_Q phase fraction of the wanted 5e-5.
Based on this temperature the following heat treatment was found:
- Starting and staying at 800C for 1 second
- Heating to 1402 with a heating rate of 100K/s
- Annealing at 1402 for 1e6 seconds
Don't forget to set the precipitation domain to virtual in the first segment!
Setting up the graphical output
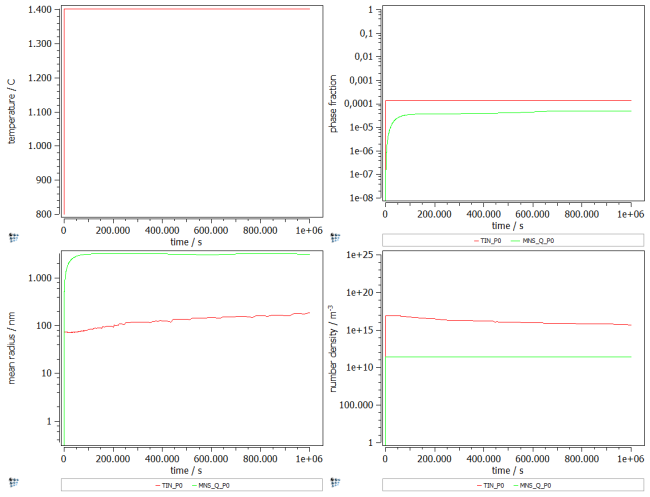
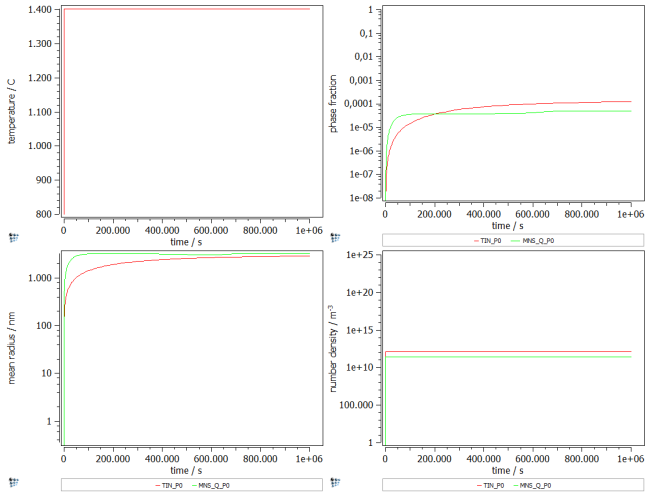
Create a p1 plot window with 4 plots and plot T$C, f_prec$*, num_prec$* and r_mean$*. Change the scaling and title of axes according to the figure below. Alternatively, run this script to set up the windows accordingly.
If you are interested in the size distribution, create a histogram and plot the primary precipitates.
Starting the simulation
As everything is configured, the simulation can begin. Open up the Precipitation simulation dialog, and select the virtual heat treatment as the temperature control. Then press Go!
First results
The first results show that the phase fraction and size of MnS_Q precipitates is at the desired values, however TiN precipitates are slightly too many, and far too small for primary precipitates.
As a next step, we try to tune the results by increasing the size, and decreasing the phase fraction of TiN precipitates.
Tuning the results
As the values for MnS_Q precipitates are already correct, we only aim on changing the TiN's values. As a first step, we change the nucleation site to grain boundary edges. This decreases the number density, and hence increases the mean radius.
However, this is not enough the reach a particle size of about 2µm. Therefore change the nucleation constant to 7e-2, and the substitutional matrix diffusion enhancement factor to 0.48.
This results in the phase fraction which was found with the Scheil calculation, as well as a particle size big enough for primaries.
#### /TIN_P0/ moles: 0,000121421, gm: -223600 (207516), sff: 0,5 Phasestatus: dormant dfm in /FCC_A1#01/: 1,41416e+003, r_ = 2,95912e-006, n = 1,10179e+012 TI +4,92560e-001 N +4,48515e-001 C +5,14850e-002 NB +7,43951e-003 #### /MNS_Q_P0/ moles: 4,98449e-005, gm: -165710 (150509), sff: 0,5 Phasestatus: dormant dfm in /FCC_A1#01/: 4,28272e+000, r_ = 3,33417e-006, n = 2,56116e+011 S +5,00000e-001 MN +4,76845e-001 FE +2,31457e-002 CU +9,09347e-006
Consecutive articles
none