Table of Contents
Example P13: NbC and AlN precipitation in the deformed steel
Compatibility
MatCalc version: 5.51.0019
Database: mc_fe.tdb (alternatively mc_x_FeAlCNNbTi.tdb or mc_sample_fe.tdb) mc_sample_fe.ddb
Author: Walter Mayer
Created: 2012-12-10
Revisions:
Objectives
This example demonstrates the influence of the plastic deformation on the precipitation kinetics. It is shown how the elastic strains are introduced into the sample heat treatment simulation. The model which describes the evolution of the dislocation density due to the applied stress is briefly discussed
Complementary files
Click here to view the script for this tutorial.
Main document
Setup thermodynamics
Set up the thermodynamics by either loading the mc_fe, the example database mc_x_FeAlCNNbTi or the free mc_sample_fe database
Select the following phases, elements and enter the chemical composition in wt-%:
| Elements | Chemical composition | Phases |
|---|---|---|
| Al | 0.05 | BCC_A2 |
| C | 0.2 | FCC_A1 |
| N | 0.005 | CEMENTITE |
| Nb | 0.04 | ALN |
Set Fe to reference element. Conclude the thermodynamic setup by loading the diffusion database for Fe. To ensure correct thermodynamic values and no driving force errors (DFM-errors), set automatic start values and calculate an equilibrium at 1500 C.
Precipitation domain and precipitates
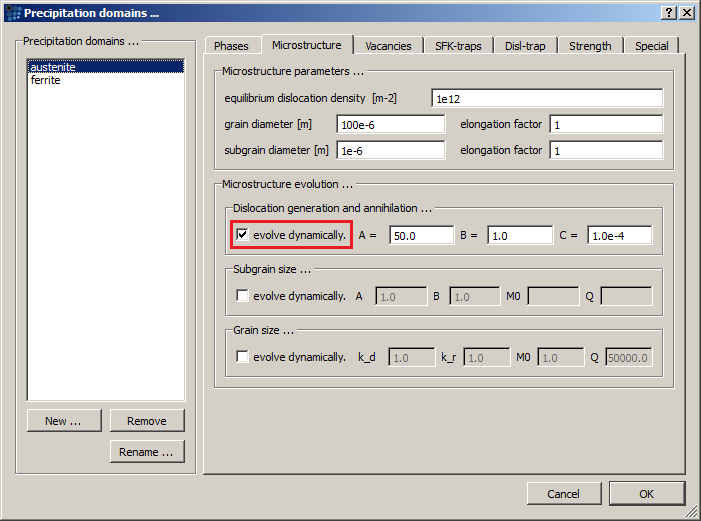
Create two precipitation domains: ferrite (with BCC_A2 matrix phase) and austenite (with FCC_A1 matrix phase). Next, activate the dislocation density evolution model for the austenite domain.
Leave the model parameters, as well as all other domain settings on default value. The implemented model was described by Sherstnev et al. 1) and is also discussed in this technical paper. Here, it will be only mentioned that effects described by the model parameters are: dislocation creation due to the deformation ('A'), anihilation by the meeting of dislocations with antiparallel Burger's vector ('B') and anihilation by dislocation climb ('C')
Follow by setting up the precipitates. Create one precipitate phase for cementite, two phases for fcc_a1#01 and four phases for AlN. Choose the settings, as given in the table below
| Precipitate | Tab | Option | Value |
|---|---|---|---|
| cementite_p0 | Precipitate | #size classes | 25 (Initialize!) |
| cementite_p0 | Attached to pd | ferrite | |
| cementite_p0 | Kinetic alias name | Cementite | |
| cementite_p0 | Nucl. sites | Nucleation sites | dislocations |
| Precipitate | Tab | Option | Value |
|---|---|---|---|
| fcc_a1#01_p0 | Precipitate | #size classes | 25 (Initialize!) |
| fcc_a1#01_p0 | Kinetic alias name | NbC(aust,d) | |
| fcc_a1#01_p0 | Nucleation | Restrict nucleation to prec domain | ✔, select austenite |
| fcc_a1#01_p0 | Nucl. sites | Nucleation sites | dislocations |
| Precipitate | Tab | Option | Value |
|---|---|---|---|
| fcc_a1#01_p1 | Precipitate | #size classes | 25 (Initialize!) |
| fcc_a1#01_p1 | Kinetic alias name | NbC(aust,gb) | |
| fcc_a1#01_p1 | Nucleation | Restrict nucleation to prec domain | ✔, select ferrite |
| fcc_a1#01_p1 | Nucl. sites | Nucleation sites | grain boundary |
| Precipitate | Tab | Option | Value |
|---|---|---|---|
| AlN_p0 | Precipitate | #size classes | 25 (Initialize!) |
| AlN_p0 | Kinetic alias name | AlN(aust,d) | |
| AlN_p0 | Nucleation | Restrict nucleation to prec domain | ✔, select austenite |
| AlN_p0 | Account for coherent misfit stress | ✔ | |
| AlN_p0 | Nucl. sites | Nucleation sites | dislocations |
| AlN_p0 | Structure | Vol. misfit | 0.27, remove ✔ from 'auto' |
| Precipitate | Tab | Option | Value |
|---|---|---|---|
| AlN_p1 | Precipitate | #size classes | 25 (Initialize!) |
| AlN_p1 | Kinetic alias name | AlN(aust,gb) | |
| AlN_p1 | Nucleation | Restrict nucleation to prec domain | ✔, select austenite |
| AlN_p1 | Nucl. sites | Nucleation sites | grain boundary |
| Precipitate | Tab | Option | Value |
|---|---|---|---|
| AlN_p2 | Precipitate | #size classes | 25 (Initialize!) |
| AlN_p2 | Kinetic alias name | AlN(ferr,d) | |
| AlN_p2 | Nucleation | Restrict nucleation to prec domain | ✔, select ferrite |
| AlN_p2 | Account for coherent misfit stress | ✔ | |
| AlN_p2 | Nucl. sites | Nucleation sites | dislocations |
| AlN_p2 | Structure | Vol. misfit | 0.27, remove ✔ from 'auto' |
| Precipitate | Tab | Option | Value |
|---|---|---|---|
| AlN_p3 | Precipitate | #size classes | 25 (Initialize!) |
| AlN_p3 | Kinetic alias name | AlN(ferr,gb) | |
| AlN_p3 | Nucleation | Restrict nucleation to prec domain | ✔, select ferrite |
| AlN_p3 | Nucl. sites | Nucleation sites | grain boundary |
Heat treatment
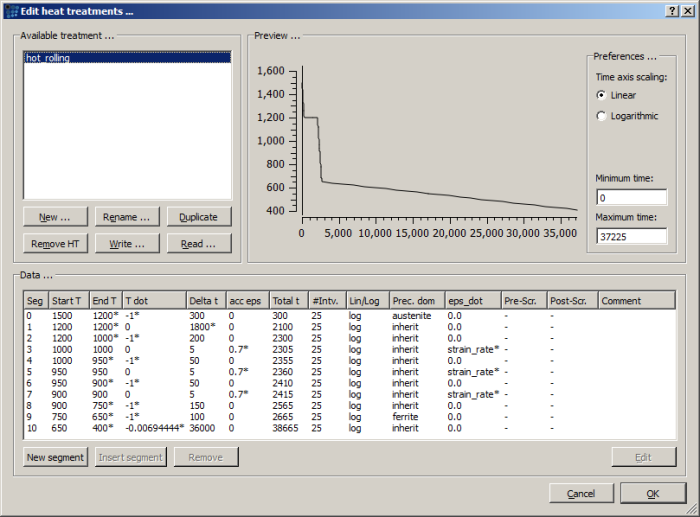
Define the heat treatment with the following segments:
- Start at temperature of 1500 C. Cool down to 1200 C with 1 C/s cooling rate. Select austenite as precipitation domain.
- Hold at 1200 C for 30 minutes.
- Cool down to 1000 C with 1 C/s cooling rate.
- Cool down to 950 C with 1 C/s cooling rate.
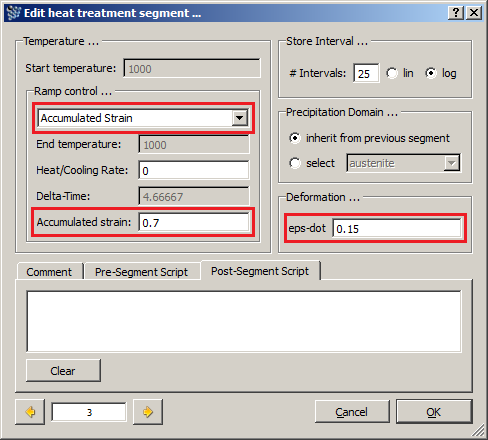
- Deform material with the strain rate of 0.15 till the accumulated strain reaches the 0.7 value. Hold the material at 950 C (cooling rate is 0 C/s).
- Cool down to 900 C with 1 C/s cooling rate.
- Deform material with the strain rate of 0.15 till the accumulated strain reaches the 0.7 value. Hold the material at 900 C (cooling rate is 0 C/s).
- Cool down to 750 C with 1 C/s cooling rate.
- Cool down to 650 C with 1 C/s cooling rate. Switch precipitation domain to ferrite.
- Cool down to 400 C within 10 hours.
Precipitation simulation
Before starting the simulation, create 4 diagrams which will describe the precipitation characteristics (temperature, phase fraction, number densities, mean radii) - this is conveniently done by clicking on 'View' → 'Create new window …' → 'user-defined' tab → '03_kinetics_4_frames_T_f_n_r_linX'. Start the simulation with the temperature controlled by the defined heat-treatment.
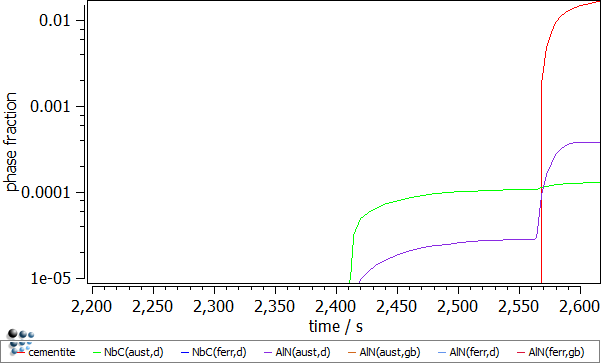
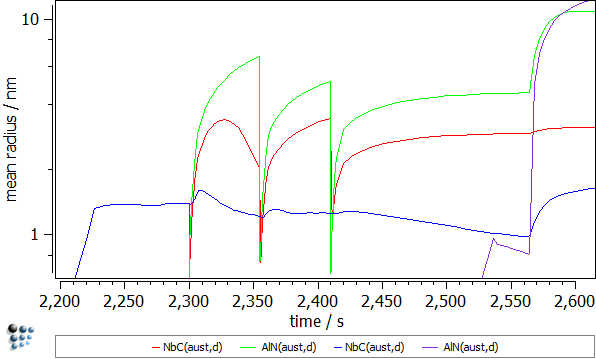
The first look on the calculation results suggests that the whole precipitation appears in the ferrite domain (after ~2560 seconds). By magnifying the time axis (default x-axis!) from 2200 to 2600 seconds, one can notice the precipitates occurring in the austenite domain already where the deformation stage was introduced.
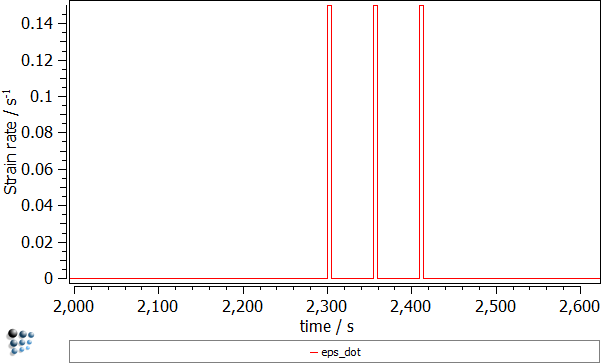
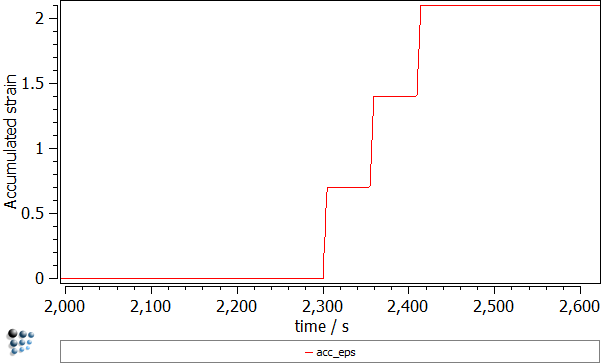
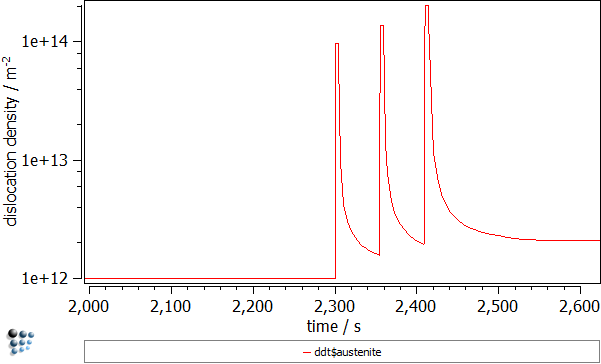
This deformation process can be illustrated by the following plots:
- Strain rate ('EPS_DOT' variable found in the 'kinetics:general' group). The three peaks correspond to the deformation segments.
- Strain accumulated in the sample ('ACC_EPS' variable found in the 'kinetics:general' group). Every deformation segment resulted in increasing the strain by 0.7.
- Dislocation density in the austenite ('DDT$austenite' variable found in the 'kinetics:pd struct' group). Again, three peaks corresponding to the deformation stages can be recognized. The dislocation density rises by two orders of magnitude.
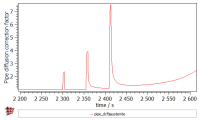
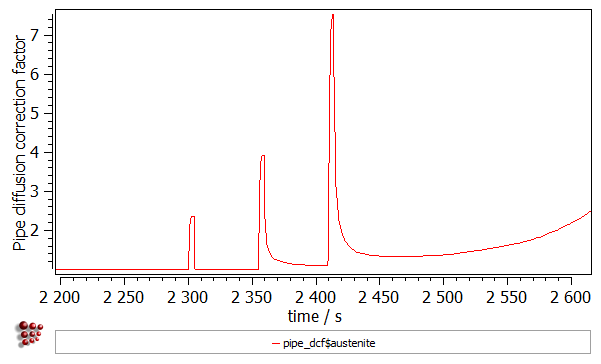
- Pipe diffusion correction factor ('PIPE_DCF$austenite$*' variable found in the 'kinetics:pd special' group). The dislocations allow for a faster diffusion of the atoms. This effect is known as “pipe diffusion” and is represented with a correction factor, by which the diffusion coefficients of elements are multiplied (the same factor is assumed for all elements). The magnitude of this factor depends on the amount of the excess dislocation density and temperature. Increasing the number of dislocations during the deformation results in the faster diffusion in the material.
The influence of the deformation on the precipitation kinetics can be discussed on the following two plots:
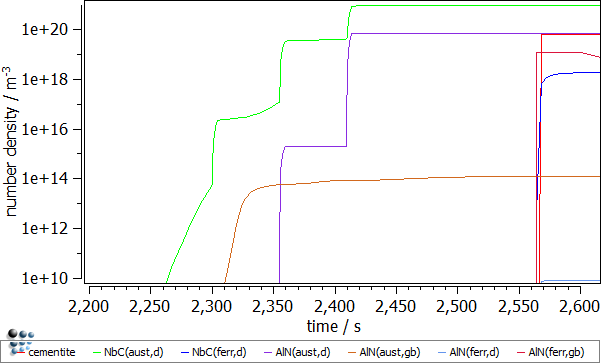
- Number density of the precipitates. The number of precipitates nucleating on dislocations in austenite is increasing significantly during the deformation segments. Three increments for NbC(aust,d) and two for AlN(aust,d) precipitates can be identified. This observation is explained by the increasing number of the dislocations and thus the increasing number of the available nucleation sites for those precipitates.
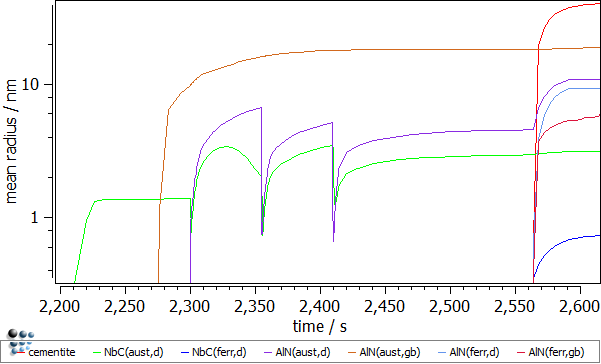
- Mean radii of the precipitates. The rapid descent of the mean radius for the NbC(aust,d) and AlN(aust,d) precipitates nucleating at dislocations is observed during the dislocation segments. This is an effect of the outburst of the new nuclei for these phases. These falls are immediately compensated by the significant radius growth which is a result of the enhanced diffusion due to the increased dislocation number (pipe-diffusion).
Comparison with no deformation case
In order to demonstrate the effects of the deformation on the precipitation process, the calculation will be repeated for the system without any deformation steps. Rename the current buffer to 'w_deform'. Create the new buffer and rename it to 'no_deform'. Duplicate and lock all series in the plots. Next, edit the segments which describe the deformation stages in the heat treatment and write zero to 'eps-dot'. In options window for the plots switch the displayed buffer to 'no_deform' and start the precipitation kinetics calculation.
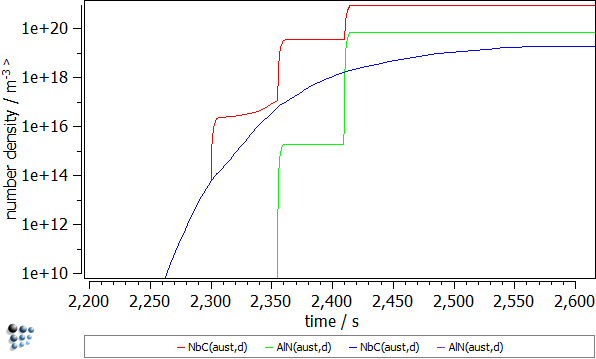
Let's compare the results for the NbC(aust,d) and AlN(aust,d) from the both simulations
- Number densities. The number densities for these phases are a few order smaller than in the deformed material. The phase fractions show a similar behavior. Noteworthy, the precipitates for AlN are virtually non-existent in the samples without strain. As expected, the excess dislocations increase the nucleation rates in the deformed material resulting in the higher number of precipitates.
- Mean radii. The differences here are not very prominent. Nevertheless, the radii in the strained samples are larger, as the introduced dislocations enable the pipe-diffusion.